Porphyrin and phthalocyanine-based thin film
Pophyrins • Phthalocyanines • Modelling
T. Heine,(a) S. Bräse,(b) C. Wöll (a)
(a) Faculty of Chemistry and Food Chemistry, TU Dresden, Mommsenstr. 13, 01062 Dresden
(b) Institute of Biological and Chemical Systems – Functional Molecular Systems (IBCS-FMS), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen
(c) Institute of Functional Interfaces, Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen
The synthesis of differently substituted porphyrin linkers were achieved using different sets of cross-coupling reactions. They were successfully embedded in novel SURMOF structures. At the beginning of the synthesis of trans-A2B2 phthalocyanines, the first goal was to obtain phthalocyanines with a better solubility than the unsubstituted or substituted phthalocyanines with small residues. This goal was achieved by the synthesis of precursors with sterically demanding residues such as long alkoxy chains or triptycene residues. The synthesis of metal-free phthalocyanines also increased the solubility, although the yields were lower. We are currently working on an approach that starts with the symmetrical octahydroxy-phthalocyanine, which will subsequently be functionalized with e.g. boronic acids. However, boronic esters strongly reduce the solubility despite sterically demanding groups. Besides the synthesis of phthalocyanines, the synthesis of other porphyrin-based molecules are achieved to increase the absorption of red light, for example tetrabenzoporphyrin and especially tetrabenzodiazaporphyrin, which has a high absorption of red light. These structures show a better solubility and the linear unit is easier to introduce.
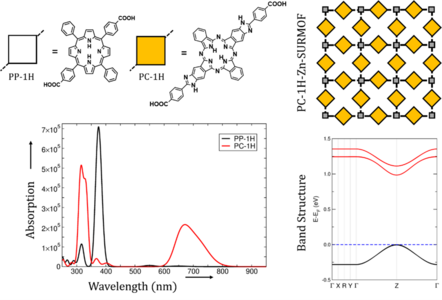
Here, we extend the approach of the first funding period to incorporate phthalocyanine (Pc) linkers as light-harvesting units. Experiments and our calculations at the TD-DFT level of theory showed that Pc-linkers show particularly strong absorption in the visible range of the solar spectrum. Our first investigation aimed at the impact of the translational symmetry and stacking on the optical properties of the Pc. As shown in Figure 1, Pc show a significantly stronger absorption in the visible range of the solar spectrum, strongly exceeding that of the Q-bands in porphyrins (PPs). This property is maintained as reflected in the band structure, calculated using hybrid density functional HSE06, which shows a rather narrow band gap. In particular, we observe a strong band dispersion of about 250 meV in both valence and conduction bands, with a direct band gap at the Z point (indicating transport normal to the lattice plane). The optoelectronic properties resulting from this remarkable band structure are currently explored.
Because of the challenges in synthesizing A2B2 functionalized phthalocyanines, a different strategy to connect the anchoring groups was employed starting from putting Si into the center of the phthalocyanine ring. The layer-by-layer SURMOF approach was successfully used to grow highly oriented SURMOFs of type SURMOF-2. A thorough photophysical characterization revealed the presence of J-aggregates. Thec excellent absorption properties of this phthalocyanine-based linkers were the used to realize the first MOF-based optical cavity with huge Rabi splittings.[3] In addition, in this study the solvatochromic shifts of the phthalocyanine optical absorption bands in different solvents were exploited to demonstrate a novel type of sensor bases on strong coupling in Fabry-Perot resonator. In future work we will build such optical cavities from A2B2 functionalized phthalocyanines.
References
(1) R. Haldar, K. Batra, S. Marschner, A. Kuc, S. Zahn, R. Fischer, S. Bräse, T. Heine, C. Wöll, Chem. Eur. J. 2019, 25, 7847-7851. Bridging the green gap: MOF hetero-multilayers assembled from porphyrinic linkers identified using computational screening
(2) S. M. Marschner, R. Haldar, O. Fuhr, C. Wöll, S. Bräse, Chem. Eur. J. 2020, in press. Modular Synthesis of trans-A2B2-Porphyrins with Terminal Esters – Systematically Broadening the Scope of Linear Linkers for Porphyrin-Based MOFs
(3) R. Haldar, Z.H. Fu, R. Joseph, D. Herrero, L. Martin-Gomis, B.S. Richards, I.A. Howard, A. Sastre-Santos, C. Wöll, Guest-responsive polaritons in a porous framework: chromophoric sponges in optical QED cavities. Chem. Sci., 2020. 11(30), 7972-7978
Interested in finding out more? Click here to learn more about this overarching COORNETs Phase II team and project.